Bone Allografts
Bone allografts are procured through aseptic explantation of whole bones or bone fragments during multi-organ procurement procedures from brain-dead deceased donors. Tissue retrieval must be completed within 12 hours post-asystole in authorized healthcare facilities, in strict compliance with the regulatory framework of the Republic of Belarus.
The processing protocol involves sequential physico-chemical treatment to eliminate cellular components and lipid fractions, including:
- Mechanical debridement and shapingPrecision cutting and washing
- Membranolysis for cellular removal
- Delipidization process
- Freeze-drying (lyophilization), Terminal sterilization via gamma irradiation
Clinical Applications:
These allografts are indicated for:
Reconstruction of bone defects resulting from:
- Traumatic injuries
- Congenital anomalies
- Acquired pathologies
Utilization across multiple surgical specialties:
- Orthopedic and trauma surgery
- Oncological reconstructions
- Craniomaxillofacial procedures
- Dental and plastic reconstructive surgery
Cancellous Bone Blocks
Product Characteristics:Tissue composition: Decellularized cancellous bone fragments devoid of soft tissue remnants
Source: Epiphyseal regions of large bones (femur, tibia)
Key properties:
- Osteoconductive matrix facilitating host tissue incorporation
- Customizable sizing for intraoperative contouring
Bone void filling in:
- Oncological resections
- Spinal arthrodesis procedures
- Sinus lift augmentation
- other clinical situations requiring bone defect repair
 |

|

|

|
|
Spongy bone block Block size: 1 х 1 х 1 см; 1 см³ |
Spongy bone block Block size: 1 х 2 х 2 см; 4 см³
|
Spongy bone block Block size: 1 х 2 х 5 см; 5 см |
Spongy bone block Block size: 1 х 3 х 5 см; 15 см³ |
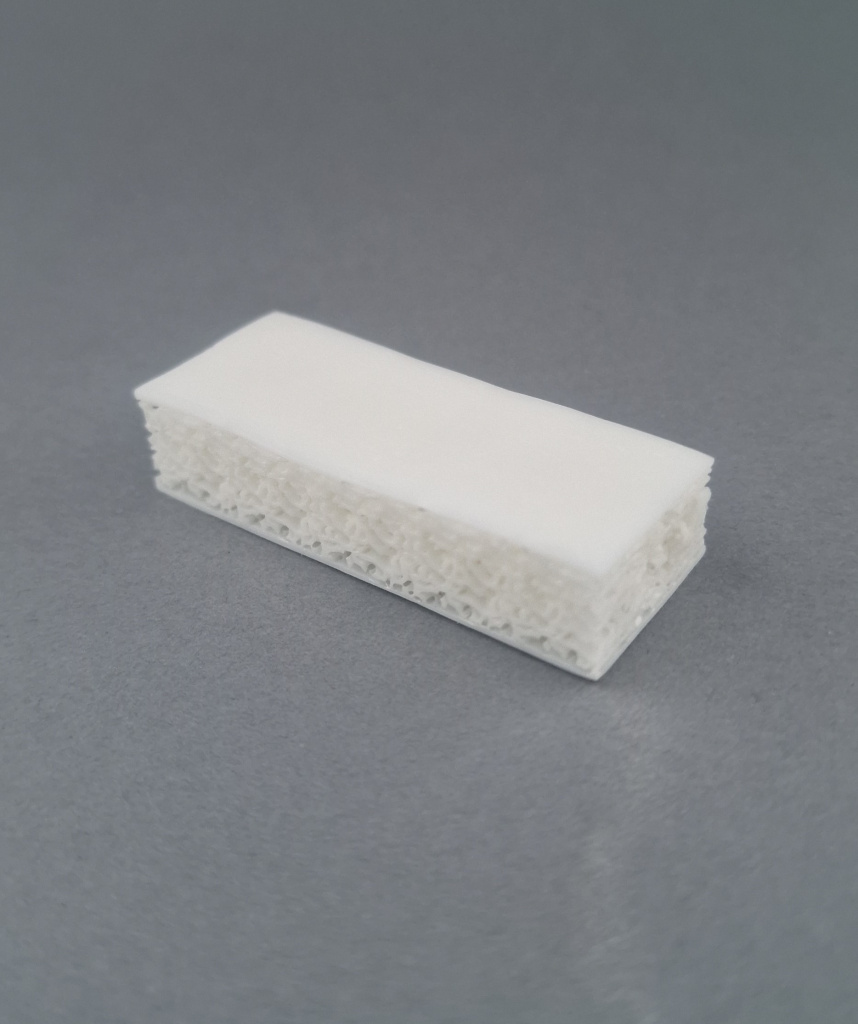
Cancellous-Cortical Bone Blocks
Tissue Composition:
- Decellularized cancellous core with cortical surfaces (1-3 sides)
- Complete removal of soft tissue and cellular elements
Anatomic Sources:
- Epiphyses of long bones (femur, tibia)
- Iliac crest
Cortical Components: Provide mechanical stability (Annex 5 - Biomechanical Testing)
Cancellous Core: Facilitates:
- Vascular invasion (Chapter 1.3.1 - Biological Integration)
- Osteoconduction
Bone void filling in:
- Oncological resections
- Spinal arthrodesis procedures
- Sinus lift augmentation
- other clinical situations requiring bone defect repair
 |

|
|

|

|
|
Monocortical iliac crest Block size: 1 х 3 х 3 см; 9 см³ |
Bicortical iliac crest Block size: 1 х 2 х 2 см; 4 см³
|
Bicortical iliac crest Block size: 1 х 2 х 4 см; 8 см³ |
Tricortical iliac crest Block size: 1 х 2 х 3 см; 6см³
|
Tricortical iliac crest Block size: 1 х 2 х 4; 8 см³ |
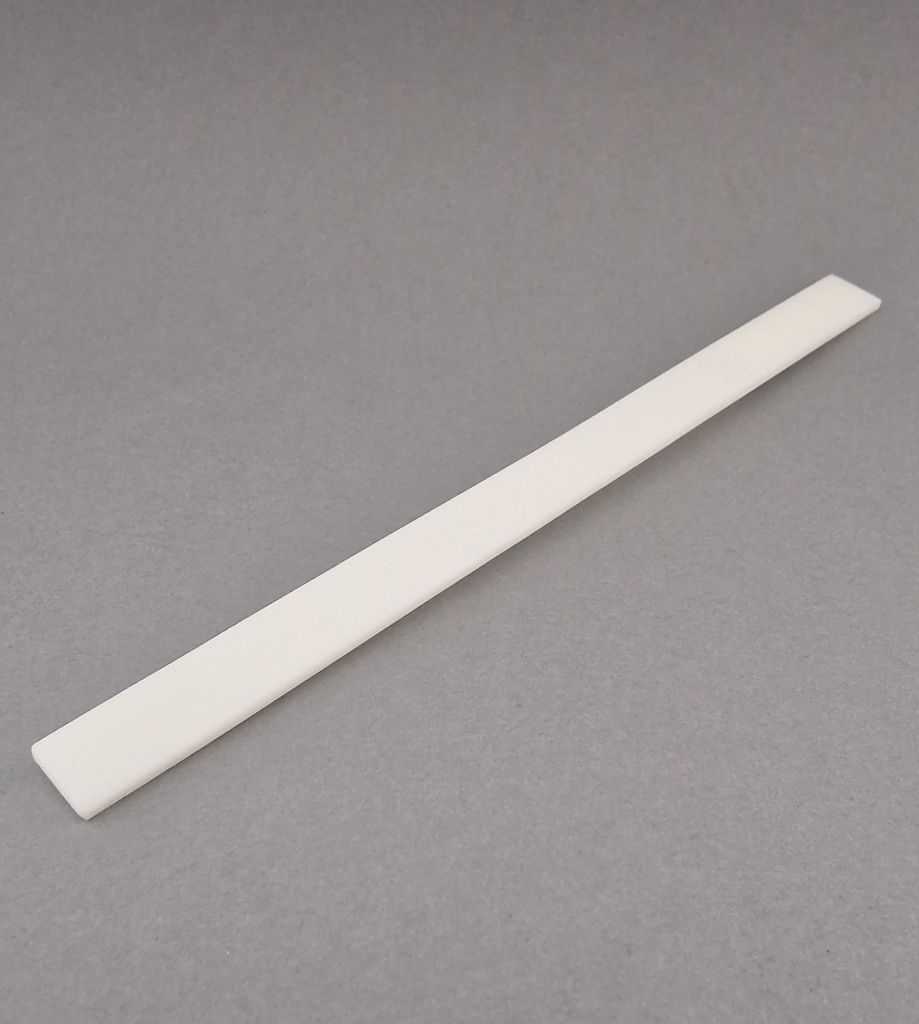
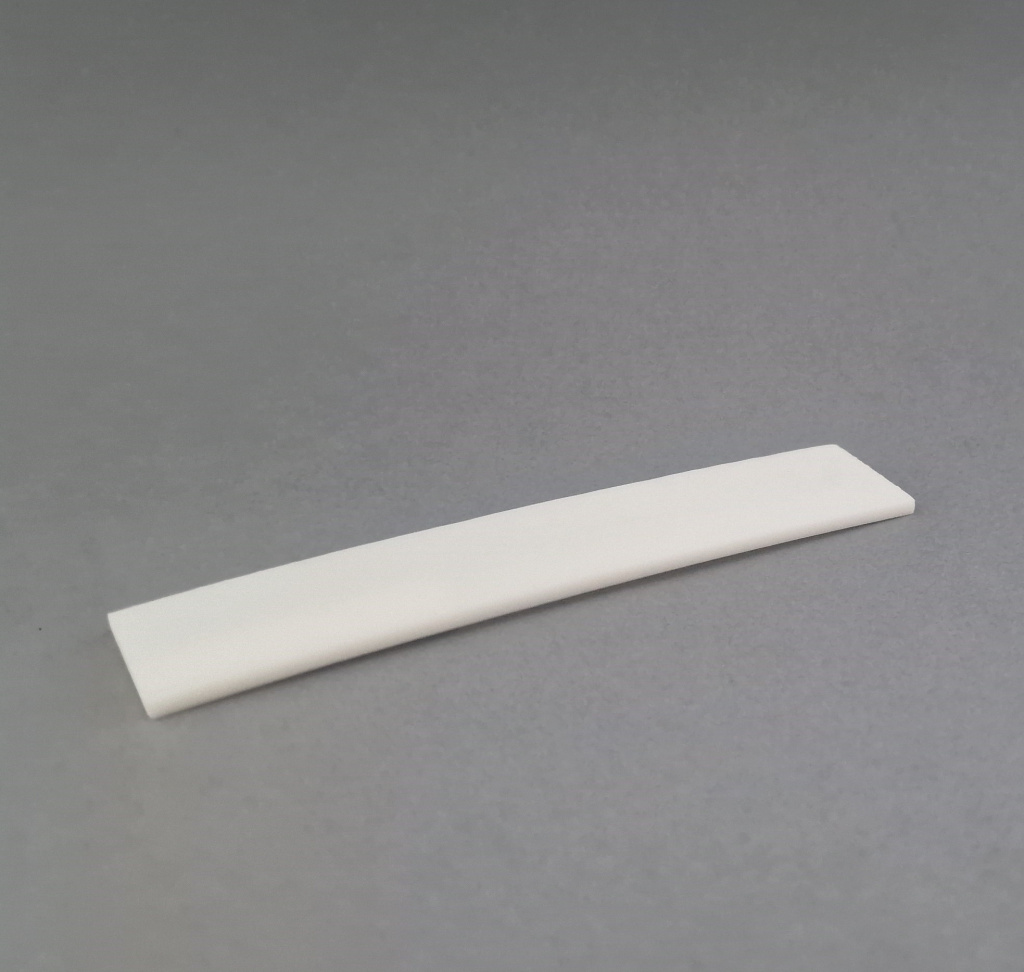
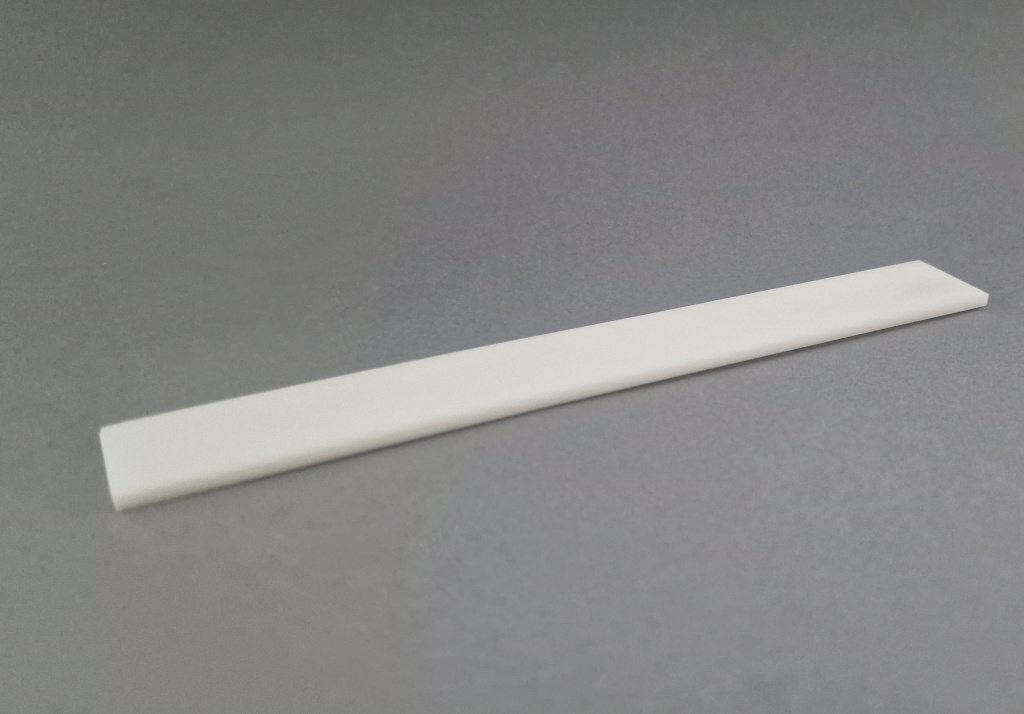
Cortical Bone Plates
Source Material: Diaphyseal cortical bone from femur or tibia
Processing Method: Longitudinal sectioning and precision machining
Key Features:
- Complete removal of cellular elements and bone marrow (Chapter 6.7.2 - Decellularization)
- High mechanical strength (Annex 5 - Biomechanical Testing)
- Preserved cortical microstructure
- Revision arthroplasty
- Resection of bone neoplasms
- Reconstructive procedures for:
- Osteomyelitis defects
- Non-union fractures
- Comminuted fracture stabilization
 |

|
 |
|
|
|
Cortical plate Block size: 1 х 5 см; 5 см2 Thickness of the cortical plate from 1 to 3 mm. |
Cortical plate Block size: 1 х 10 см; 10 см2 Thickness of the cortical plate from 1 to 3 mm. |
Cortical plate Block size: 1 х 15 см; 15 см2 Thickness of the cortical plate from 1 to 3 mm. |
Cortical plate Block size: 2 х 10 см; 20 см2 Thickness of the cortical plate from 1 to 3 mm. |
Cortical plate Block size: 2 х 15 см; 30 см2 Thickness of the cortical plate from 1 to 3 mm. |
Bone Powder
Source Material:
Cancellous and/or cortical bone from:
- Iliac crest
- Lower limb epiphyses
Particle Characteristics:
- Size range: 0.1-2.0 mm (calibrated sieving)
- Options:
- Pure cancellous particulate
- Pure cortical particulate
- Custom blends
Clinical Applications
- Sinus lift procedures
- Alveolar ridge preservation
- Post-extraction socket grafting
- Defect filling in orthopedic cases
- Craniofacial reconstruction
 |

|
|
Cancellous powder
Quantity: 1 g; 2 g |
Cancellous-cortical powder 50/50 Cancellous-cortical powder 80/20
Quantity: 1 g; 2 g |
Bone Chips
Source Material:
Cancellous/cortical bone from:
- Iliac crest
- Lower limb epiphyses
Particle Characteristics:
Size: 2.5-8.5 mm (mechanically milled)
Composition options:
- Pure cancellous
- Pure cortical
- Custom blends
Clinical Applications
- Revision arthroplasty
- Tumor resection defects
- Fracture void filling
- Pseudarthrosis treatment
- Spinal arthrodesis
 |

|
|
Cancellous chips Quantity: 10 g 20 g 30 g |
Cancellous-cortical chips 50/50 Cancellous-cortical chips 80/20
Quantity: 10 g 20 g 30 g |




